The inherent coupling of charge transfer and mass transport processes: the curious electrochemical reversibility
The paper authored by
R. Seeber,
C. Zanardi and
G. Inzelt
is published in Chemtexts: The Textbook Journal of Chemistry (2016, vol. 2, pp. 1–16).
Abstract:
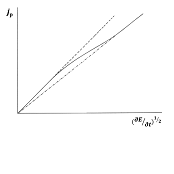
As a complement to a previous contribution from us, the mass transport mechanisms of the electroactive species to and from the electrode in an uncomplicated electrode mechanism are considered. The electrode process as a whole is discussed, with emphasis to its reversibility degree, as results from the relevant responses in controlled potential techniques, such as chronoamperometry and current sampling voltammetry, linear sweep and cyclic voltammetry, and in rotating disk voltammetry. The electrode process as a whole, composed by charge transfer and mass transport steps that concur to condition the current flowing, is analysed on the basis of the relative rates of the two steps, as well of the time window within which the process is observed. The so-called ‘boundary value problem’ for uncomplicated charge transfers with different reversibility degrees is outlined. Supplementary Material is available, in which the simulated concentration profiles for reduced and oxidised species reacting at an electrode, at which a triangular potential waveform is applied, are linked to the corresponding current densities.
